[ Instrument R & D of Instrument Network ] With the rapid development of modern industry, the development of high-efficiency precision separation materials and technologies to achieve accurate separation of ion and molecular scales will have a transformative impact on energy, water, chemical, pharmaceutical and other fields. Membrane separation has the advantages of low energy consumption, mild separation conditions, and easy operation. It has been widely used in various fields of industrialized production and daily life. However, the preparation of nanoporous membrane materials with a highly uniform pore size and the precise separation of ions or small molecule compounds still face great challenges. In order to achieve this goal, a series of new membrane materials based on one-dimensional nanomaterials (such as carbon nanotubes), two-dimensional nanomaterials (such as graphene, etc.) and aquaporins have been developed in recent years. Membrane materials still face problems in large-scale preparation, poor long-term stability, and high cost in practical applications.
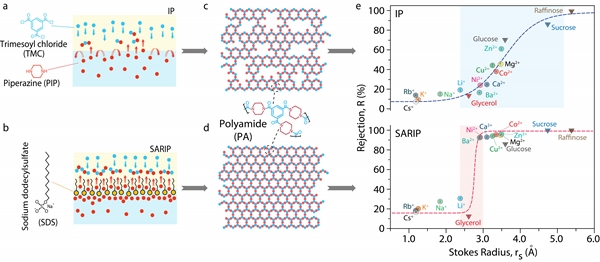
Recently, the research team of Jin Jian, a researcher at the Institute of Nanotechnology and Nanobionics, Chinese Academy of Sciences, and Lin Shihong, a professor at Vanderbilt University in the United States, designed and developed a self-assembled ordered monomolecular membrane that uses surfactants to regulate the interfacial polymerization process ( Surfactant-assembly regulated interfacial polymerization (or SARIP) is a strategy to prepare thin-film composite nanofiltration membrane (TFC-NF) with ultra-narrow pore size distribution, which achieves high-precision separation of molecules / ions at subangstrom level.
Interfacial polymerization: The polycondensation reaction performed on the interface (or the organic phase side of the interface) of two solutions that are incompatible with each other and in which two monomers are dissolved is called interfacial polymerization. The polymer obtained by the reaction is insoluble in the solvent and precipitates at the interface. Interfacial polymerization is suitable for irreversible polycondensation reactions. The requirements for monomer purity and quantity ratio are not high, the solvent consumption is large, the equipment utilization rate is low, and it can be used for ester high-melting polymer polyamide, polycarbonate, etc.
The interfacial polymerization method is currently an effective method for large-scale preparation of TFC-NF, and is also the main method for industrial nanofiltration membrane production. In the traditional interfacial polymerization reaction, the diamine (PIP) monomer dissolved in the water phase diffuses into the oil phase containing the acid chloride (TMC), and a polymerization reaction occurs at the water-oil interface to form a polyamide separation layer. Since the reaction of these two monomers is an ultra-fast process with high reactivity, and the diffusion of PIP at the water / oil interface is an uncontrollable random process, the resulting polyamide separation layer has an uneven structure and a relatively wide pore size distribution. It is difficult to achieve high-precision screening of molecules or ions of similar size.
Polyamide thin film chromatography is a new chromatography technology developed after 1966. Because it has the advantages of high sensitivity, strong resolution, fast and easy operation, it has been widely used in the analysis of various compounds.
The adsorption of polyamide on polar substances is due to its ability to form hydrogen bonds with the separated material. The strength of this hydrogen bond determines the adsorption capacity between the separated material and the polyamide film. During chromatography, the spreading agent competes with the separated product on the surface of the polyamide membrane to form hydrogen bonds. Therefore, selecting the appropriate spreading agent to make the separation material on the surface of the polyamide membrane adsorption, desorption, re-adsorption, re-desorption continuous process, so that the separation material can achieve the purpose of separation.
Taking Li + (Stokes radius: 2.4 angstroms) and Ba2 + (Stokes radius: 2.9 angstroms) as examples, the membrane rejection rates for them were 19% and 17%, respectively, and separation was almost impossible. In this work, they introduced a self-assembled ordered monomolecular film formed by anionic surfactant-sodium dodecyl sulfonate (SDS) at the oil-water interface. The ordered monomolecular film greatly changed and regulated The cross-interfacial diffusion behavior of PIP monomers.
The specific performance is as follows: 1. The negatively charged sulfonic acid group and weakly positively charged PIP molecule form a certain electrostatic attraction interaction in the SDS, so that the PIP monomer is pre-enriched on the sulfonic acid group side of the SDS before diffusing The oil phase, which makes the distribution of PIP at the interface more uniform, and the increased concentration gradient of PIP monomer in the water / oil phase further increases its diffusion rate, which is conducive to the formation of a higher degree of cross-linking polymer. Amide separation layer; 2. The presence of SDS ordered monomolecular membrane can effectively regulate the disordered diffusion of PIP monomer at the water / oil interface. At the same time, both molecular dynamics and first-principles calculations show that PIP monomer follows The energy barrier to be overcome for the transport of ordered monomolecular films across interfaces is lower.
That is to say, the ordered monomolecular membrane promotes the rapid and uniform occurrence of interfacial polymerization, and the resulting polyamide separation layer has a narrower distribution of uniform pore diameters. The obtained membranes had rejection rates of Li + and Ba2 + of 30% and 93%, respectively, and showed obvious sieving and separation performance, and their separation accuracy reached Angstrom grade.
SARIP has proved to be an effective strategy for regulating monomer expansion behavior to regulate interfacial polymerization reaction and obtain thin-film composite nanofiltration membranes with ultra-narrow pore size distribution and ultra-high precision separation performance, which is based on the preparation of existing industrial nanofiltration membranes Process technology makes it possible for large-scale preparation, with potential practical application value and prospects.
The advantages of nanofiltration membrane:
1. The concentration and purification process is carried out at room temperature, without phase change, no chemical reaction, does not bring in other impurities and cause decomposition and denaturation of the product, especially suitable for heat sensitive substances.
2. It can remove the salt of the product, reduce the ash of the product, and improve the purity of the product. Compared with the desalination of the solvent, not only the product quality is better, but the yield can be improved.
3. The process yield is high and the loss is low 4. The acid, alkali, alcohol and other effective substances in the solution can be recovered to realize the recycling of resources
5. The structure of the equipment is compact, the floor space is small, and the energy consumption is low
6. Easy to operate, can realize automatic operation, good stability and easy maintenance.
network enclosures,network enclosures,network plastic enclosure,Network Racks
wybox inc. , https://www.wybox-inc.com
